Daniel Erlacher & Michael Schredl, published in the International Journal of Dream Research Volume 1, No. 1, 2008
Summary
This paper addresses the correlation of dreamed and actual actions. This issue is related to the theory of neural simulation of action. The simulation theory postulates that, in general, covert actions are actual actions relying on the same brain regions, except for the fact that they are not executed. By reviewing studies conducted in the field of dream and lucid dream research on REM sleep it will be shown that correlations between dreamed and actual actions can be found for central nervous activity, autonomic responses and time aspects.
Recent findings from research on lucid dreaming and motor learning further support the notion that actions in dreams are represented on higher cognitive levels - equivalent to actual movements - and therefore share, to some extent, the same central structures. The reviewed findings will be discussed and future directions will be given.
1. Introduction
Dreams can be considered as a kind of imagination. Both dreaming and imagination are a simulation of the real world on a higher cognitive level. In a research report by Decety (1996) the question was addressed whether imagined and executed actions share the same neural substrate. Decety presented three methods to test this hypothesis that there is equivalence between imagined and executed motor actions: measuring central nervous activity, monitoring autonomic response and using mental chronometry. It was found that central nervous activity observed during programming and preparations of actual movements are also active during mental simulation of movement (cf. Roland, Larsen, Lassen, & Skinhoj, 1980), autonomic responses during imagery of physical activity parallel the autonomic responses to actual exercise (cf. Decety, Jeannerod, Germain, & Pastene, 1991), and the timing of imagined and actual motor actions are closely related (cf. Munzert, 2002). The data presented by Decety provided converging support for the hypothesis that mental simulation of motor actions to some extent share the same motor representations and central neural mechanisms that are used to execute actual actions (Decety, 1996; Jeannerod, 1994).
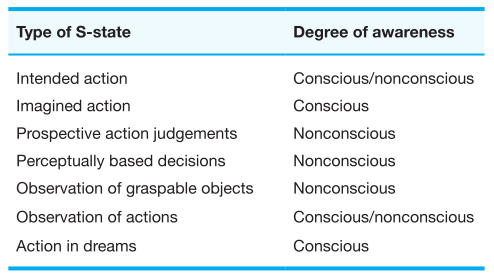
Jeannerod (2001) integrated these findings into the theory of neural simulation of action. The simulation theory postulates that, in general, covert actions are actual actions, except for the fact that they are not executed, but share for example the same cortical areas. Jeannerod terms the expression S-states to designate those “mental” states which involve action content and brain activities which simulate the same, executed action (see Table 1). For this article the focus is on imagined actions and dreamed actions, whereas the other types of S-states are of minor interest. Even though actions in dreams are explicitly mentioned as S-state by Jeannerod no empirical data has been provided in his works. This gap will be filled by this paper and the question will be raised if dreamed and executed actions in fact share the same neural substrate, e.g. that dreamed action is equivalent to real action as far as the underlying brain mechanisms are concerned. Similar to the research report from Decety (1996) evidence from several dream research domains will be reviewed.
In this paper, the focus will be on REM dreams because REM sleep is accompanied by the most vivid dream experiences. Already in an early study from Dement and Kleitman (1957) it was demonstrated that up to 80 % of the participants waken up from REM sleep remembered a vivid dream whereas only 20 % of the participants waken up during NREM sleep could report a dream. Even though it was shown later that also during NREM sleep dreams can occur to a higher percentage (e.g. Foulkes, 1962) the dreams in REM sleep are more intensive in quantity and quality, because the brain is very active during this sleep stage (e.g. Niedermeyer, 1987). Furthermore the sleeping body is paralysed throughout REM sleep by a mechanism in the brain stem (cf. Jouvet, 1979) preventing the sleeping body to act out the dreamed action. Finally, most research efforts have focused on REM dreams.
In addition to the findings in normal REM dreams, research from lucid REM dreams will also be included. The term lucid dream designates a dream in which the dreamer – while dreaming – is aware that she/he is dreaming and she/he can consciously influence the action in the dream (cf. Schredl & Erlacher, 2004). Lucid dreams occur to a high percentage in REM sleep (Erlacher, 2005). The advantage of lucid dreaming is that lucid dreamers are able to execute pre-arranged tasks in their lucid dreams and mark the beginning and the end of the task with eye signals in the electrooculogram (EOG) recording (for an example see Figure 1 and 2).
Table 1. Proposed Taxonomy of Behaviorally Defined S-States adopted from Jeannerod (2001).
The studies in this field follow basically the same design. Physiological parameters (e.g. heart rate, respiration rate) are recorded throughout REM sleep. The subject will be awakened from REM sleep and asked to report the dream content. Afterwards the dream reports will be analysed by blind raters for specific characteristics (e.g. dream emotion) or sorted into different classes (active vs. passive dream content). Then, the physiological data will be compared with the psychological data from the ratings and, finally, statements about psychophysiological correlation can be made. For lucid dream research the approach is quite similar with the exception that lucid dreamers are instructed before the night to perform specific tasks in the lucid dream and mark the beginning and the end of these tasks by eye movement signals. Trained lucid dreamers can remember pre-sleep instructions and carry out experiments during their dream. This paradigm allows precise matching of the dreamer‘s subjective reports with recorded physiological responses.
The present article reviews the major psychophysiological studies from dream and lucid dream research which support the notion that dreamed actions are a S-state in terms of theory of neural simulation of action (Jeannerods, 2001). The studies will focus on central nervous activity, autonomic responses and time aspects during lucid dreams. Further evidence for the hypothesis that dreamed actions are equivalent to actual actions will be provided by recent studies in sport science. There is growing evidence that lucid dreams can be used to practice motor skills during sleep and that this practice has positive effects on performance in wakefulness. Even though there are only a few studies in this research field the findings strongly support the hypothesis that dreamed actions are S-states because the dreamed performance has a direct effect on the same action during wakefulness. This fits well into the notion that both, dreamed and actual actions, share the same neural networks and that the neural networks were improved by the practice in lucid dreams (c.f. Erlacher, 2005).
2. Central Nervous Activity
2.1 Lesions in the brain stem
During REM sleep the general muscle tonus is actively inhibited by the REM sleep generating area in the brain stem (e.g. Dement, 1974). This physiological mechanism seems to serve a biological function in preventing the sleeping person to act out her or his dreams. This psychophysiological de-coupling between dream content and muscle activity provides a first strong argument that REM dreams can have a high correlation to corresponding physical activity, otherwise the muscle tone blockade would not be necessary. In an experiment by Jouvet and Delorme (1965) the mechanism for this muscle atonia in the brain stem was experimentally damaged in cats. The lesions resulted in overt behaviour in the animals when REM sleep occurred. These animals showed aggressive behaviour, prey catching behaviour, hygienic behaviour, etc. The actions shown by the animals did not interfere with external sources (e.g. food) and therefore it can be assumed that they were acting out their dream experience (Jouvet, 1979, 1994). The brain region responsible for muscle atonia is located in the formatio reticularis, a dense neural network which crosses with several different nucleus from the vertical brain stem. Additionally, the efferent nerves regulate the tonus in the skeletal muscles (c.f. Birbaumer & Schmidt, 1996). In addition to animal experiments, further evidence is provided by case reports of patients suffering from REM sleep behaviour disorder (RBD), in those patients the atonia is partially not functioning (Schenk & Mahowald, 1996). The following case vignette illustrate this sleep disorder.
(REM sleep behaviour disorder; man, 67 years old): “I was a halfback playing football, and after the quarterback received the ball from the center he lateraled it sideways to me and I’m supposed to go around end and cut back over tackle and – this is very vivid –as I cut back over tackle there is this big 280-poind tackle waiting -, so I, according to football rules, was to give him a shoulder and bounce him out of the way, supposedly, and when I came to I was standing in front of our dresser and I had knocked lamps, mirrors, and everything off the dresser, hit my head against the wall and my knee against the dresser.” (Schenck, Bundlie, Ettinger, & Mahowald, 1986, p. 294)
The dream example underlines that dreamed actions were directly acted out by the sleeping body. The fact that the person was actually dreaming can be inferred by the report because the person hit the dresser which was not present in his dream. RBD is a rather seldom disorder and little is known about the underlying neural mechanisms (Schenk & Mahowald, 2002). A strong association between RBD and extrapyramidal disorders have been reported (Schenk & Mahowald, 2002) and impaired striatal dopamine transporter function was found in RBD patients (Eisensehr et al., 2000), but, the critical question as to what brain mechanisms underlie REM motor dyscontrol is still uncertain. RBD should not mixed up with sleepwalking which occurs during NREM sleep; after sleepwalking the patients show a amnesia for their behaviour after waking up from their sleep and are very often not able to report any dream content (c.f. Keefauver & Guilleminault, 2000)
2.2. Dream content and EMG activity
In normal persons the sleeping body is inhibited and dreamed actions show no overt gross movements (Schredl, 2000). Despite the general muscle blockade, electrical impulses can be measured in the limb muscles which might cause small twitches (Dement, 1974). Several studies (Dement & Wolpert 1958; Wolpert 1960; Gardner et al. 1975) have shown that dreamed physical activities correspondence with the EMG activities of the sleeping body. Wolpert (1960) demonstrated that dreams in which the participants reported a lot of dreamed body movements are associated with an elevated EMG activity in arms and legs. The studies of Grossman et al. (1972) and Gardner, Grossman, Roffwarg and Weiner (1975) extend the findings from Wolpert by differentiating between dreamed arm and dreamed leg activity and by analyzing the limb EMGs. MC Guigan and Tanner (1971) have measured chin and lip EMG during REM sleep and demonstrated that dreams with talking were accompanied by elevations of EMG activity in chin and lip muscles. This finding was confirmed by Shimizu and Inoue (1986). Based on research studies investigating the expression of emotions in the waking state, Gerne and Strauch (1985) recorded EMG potentials of the corrugator (brow lowerer, negative emotions) and of the zygomatic (lip corner puller, positive emotions). In the study from Gerne and Strauch (1985) statistically significant relationships between EMG potentials and dream emotion were obtained, but a replication study from Hofer (1987) failed to do so. Overall, the findings indicate that the EMG recordings correspond to the activity reported by the dreamer during REM sleep.
Several studies with lucid dreamers (Fenwick et al., 1984; Hearne, 1983; LaBerge, Nagel, Dement and Zarcone, 1981) also demonstrated a close correlation between dreamed limb movements and EMG activities in the corresponding limb. LaBerge, Nagel, Dement, and Zarcone (1981), for example, observed that a sequence of left and right fist clenches carried out in the lucid dream resulted in a corresponding sequence of left and right forearm twitches as measured by EMG. Fenwick et al. (1984) showed that movements carried out in a lucid dream with distal muscle groups (e.g. hand) the EMG activity was higher than movements which were performed with proximal muscles (e.g. shoulder). In proximal muscles the EMG activity can disappear at all. In several case reports from Erlacher (2005) it was shown that the EMG activity from the forearm of a lucid dreaming person can change during a series of dreamed hand movements (see Figure 1). The findings in lucid and non-lucid dreams indicate that physiological mechanisms inhibit most of the activity of the skeletal muscles that otherwise would be present due to dreamed movements.

Figure 1. Recording of a REM lucid dream. The task for the participant was to carry out hand clenches with the right hand. In his dream report the subject stated that he continuously performed hand clenches between LRLR 2 and LRLR 3, but the EMG activity is changing throughout the recording partially disappearing at all (from Erlacher, 2005)
2.3. Dream content and eye movements
In dream research the relationship between dreamed eye movements and eye movements measured at the sleeping body by EOG is known as „scanning-hypothesis“. Already Ladd proposed in the year 1892 that the eyes are moving during dreaming in a similar way as they do in the waking state. In modern dream research two approaches were selected to test this hypothesis. First, global measures of visual dream activity reported by the dreamer were correlated with the number of eye movements occurring in the EOG recording prior to the awakening. Second, it was attempted to directly relate the EOG pattern to the dreamed gaze shifts.
Dement and Wolpert (1958) scored 105 dream reports whether the last sequence was visual active (e. g. looking around) or visual passive (e. g. looking at a distant object). In a similar way, they classified the EOG recordings of the REM period prior to the awakenings. These independently made classifications were correctly matched in 74 % of the ratings. Although several subsequent studies replicated this finding, other studies were not able to detect substantial relationships between visual dream content and global measures of eye movement activity (e. g. Firth & Oswald, 1975). The mixed results might be explained by methodological issues like averaging over distinct time periods to derive global scores for visual dream activity and eye movements. The averaging over time might conceal underlying and substantial correlations.
In a study by Roffwarg et al. (1962) the following approach has been chosen to enable a direct matching of dream content and eye movements. Shortly after awakening the subjects were interrogated by an interviewer who had no access to the EOG recordings of the prior REM period, about the last 10 to 20 seconds of the dream experience. This interviewer who was familiar in relating particular actions to the corresponding eye movement patterns predicted the eye movements which he would expected to occur due to the elici ted dream report. The following example from Roffwarg et al. (1962, p. 240) gives an impression of this approach.
“The last thing I remember is looking down at a small piece of paper, held at about chest level, trying slowly and haltingly, dwelling on each word, to translate something that looked like 3 lines of French poetry. It took about 20 or 30 seconds to do it, probably. I don‘t remember if I looked up at any time from the paper. As I remember, essentially, I kept my eyes on the paper.“ Interrogator’s prediction-“There should be relative REM quiescence with the exception of a few spaced leftward glances if the subject has finished a line of reading and return to the next line“. The fact that the interviewer did not predict small eye movements (reading word by word) can be explained by the impossibility to detect such eye movements or the position of the eyeballs by the commonly used a. c. amplifiers. The corresponding EOG pattern showed three small but rapid eye movements to the left (22 seconds, 8 seconds and 0.5 seconds prior to the awakening). Overall, 80 % of the clear recalled dream actions corresponded with the EOG recording. Subsequent research confirmed small but substantial relationships (Herman et al., 1984).
Another interesting approach was adopted by Dement and Kleitman (1957). They have awakened their subjects after recording a distinct EOG pattern, for example, solely horizontal or vertical eye movements or the lack of any eye movements. One subject who was awakened after vertical eye movements dreamed of standing at the bottom of a tall cliff and looking up at climbers at various levels. In a dream reported after a REM period with horizontal eye movements the subject was watching two peoples throwing tomatoes at each other. A quiet eye movement pattern was associated with driving a car (looking ahead) in the dream.
The results from dream research showed promising results, but it is, however, still not clear how tight this relationship is, i. e. whether all eye movements are related directly to the dream gaze shifts. Additionally, methodological issues have to be kept in mind, for example, the electric measurement of eye movements occurring during sleep, and that similar research areas, for example, relating eye movements to waking imagery, encounter the same difficulties matching subjective reports with actual eye movements. This may be explained by the fact that the most eye movements are carried out without consciousness, for example, looking at a small object, glances during a conversation.
In lucid dreams, a strong correlation between deliberately carried out dream gaze shifts and the corresponding eye movements of the sleeping body measured by EOG has been demonstrated (Erlacher, 2005). Already in the first sleep laboratory studies about lucid dreams from Hearne (1978) and LaBerge (1980) eye movements were used to validate lucid dreaming. In those studies the lucid dreamers were instructed to perform specific eye movements in their lucid dreams (e.g. look left, right, left, right). In the corresponding EOG recording the specific eye movement pattern can be found by up and down lines. Figure 1 and 2 depicts two examples for typical lucid dream signals. In a study by LaBerge and Zimbardo (2000) it was shown that dream gaze is more closely related to perception than to imagination. Participants were instructed to carry out a specific tracking protocol were they had to trace a circle with their stretched arm and follow the tip of the finger with their gaze. The participants performed this task during wakefulness, in waking imagination and during lucid dreaming. For all conditions the eye pattern was recorded by EOG. The analysis of the saccadic eye movements could clearly discriminate for both dreaming and perception from imagination by showing more saccadic movements for imagination than for perception and dreaming. The authors interpret their results, that dream gaze is more related to perception in wakefulness than to imagination. The study furthermore demonstrated that even smooth tracking eye movements in lucid dreams lead to corresponding activities in the EOG recording.
2.4. Dream content and EEG activity
Several dream studies investigated the question whether corresponding brain areas are active during dreaming specific actions in the same way as they do in the waking state. Zadra and Nielsen (1996) analysed the EEG recordings of dreams with strong negative affects and found higher EEG activity for the central and occipital cortex area. The EEG activity was analysed by Fourier transformation. In general, a decrease of alpha power is assumed to indicate an activated cortical area with an increase in the excitability level of the neurones (c.f. Pfurtscheller, 1992). Zadra and Nielsen (1996) assumed that this higher activity was caused by the intense pictures (occipital area) and the movements in the dream (central area), e.g. running away from unfriendly dream characters. The occipital area of the cortex correspond to the primary visual cortex and the central area of the cortex is the primary motor area a brain region corresponding to motor commands. In a single participant, Etevenon and Guillou (1986) found an alpha power decrease over the left central cortical areas in correspondence with dreamed performance of the right hand. The decrease of the alpha power over the left central brain areas can be interpreted as an activation of the primary motor cortex whereby the left hemisphere controls the right body side and vica vers.
A study by Hong et al. (1996) investigated the relationship between talking and listening during the dream and the EEG. The advantage to utilise these activities in the context of measuring EEG is the relative good localization of the corresponding brain areas (Broca‘s area: talking and Wernicke‘s area: listening). The results confirmed the hypothesis that the lower the alpha power on the left sides of those areas corresponding to Broca‘s and Wernicke‘s area was, the more expressive or receptive language was present in the dream.
In lucid dream research only a few studies have been done. Holzinger, LaBerge, and Levitan (2006) explored differences in EEG recordings between lucid and non-lucid dreams in REM sleep. One finding indicated a higher activation of the left parietal lobe for lucid dreams in comparison to non-lucid dreams. Holzinger et al. (2006) stated that this brain area is considered to be related to semantic understanding and self-awareness and therefore reflects the self awareness of the dreaming person in lucid dreams. The lucid dreamers in a study by LaBerge et al. (1981) were instructed to perform two simple cognitive tasks (counting vs. Singing) in their dreams. The analysis of the EEG alpha power showed higher activation of the left hemisphere during counting and higher activation of the right hemisphere during counting. A pattern one would expect also for wakefulness. In a study by Erlacher, Schredl and LaBerge (2003) a single participant was instructed to carry out hand clenching either with the right or the left hand or – as a control condition – counting. Those events had to be marked by eye movements allowing to analyse the EEG alpha power over the motor cortex (C3, Cz and C4) for the left or right hand movements. Results showed that EEG alpha band over bilateral motor areas decreased while the lucid dreamer executed left or right hand clenching in contrast to dream counting.
In a recent study Strelen (2006) was able to evoke event related potentials during lucid dreams by using an odd-ball paradigm with an acoustic modality. The participants were listening the night two types of short tones (high vs. low tone). The lucid dreamers were instructed to “listen” for the high tones and signal by a single left right eye movement such an event. The analysis of three lucid dreamers who were able to accomplish this experiment showed a P300 activation in the EEG analysis. The evoked potential (P300) can be interpreted as a conscious processing of the acoustic information during the lucid REM dreams, an EEG pattern which would also appear during wakefulness.
To summarize, a relationship between dream content and brain activity measured by EEG was demonstrable. It is, however, not clear how direct this relationship is, i. e. whether all dreamed activities are related directly to the EEG, especially in the non-lucid dream studies it is difficult to coorespond the dream content to the EEG recording.

Figure 2. Recording of a correctly signaled lucid dream: Five clear left-right-left-right eye movement signals are shown in the EOG channel. Typical for REM sleep: EEG channel shows low voltage mixed frequency and the muscle tone in the EMG channel is very low. See text for the corresponding dream report. Obviously the respiration rate and heart rate increase while performing squats in the lucid dreams (Erlacher & Schredl, in Press).
2.5. Dream Content and fMRI, PET
Up to now there are only few studies applying functional magnetic resonance imagery (fMRI) have been carried out with participants sleeping in the scanner. One of the reasons is that the head of the participant has to be kept in the exact same position while sleeping. Beside the uncomfortable sleeping position, the scans are causing a strong noise during the recording session (up to 80db) that interferes with falling asleep and wake up participants who had fallen asleep. Furthermore, it was not possible to do fMRI and EEG recordings simultaneously because of the strong magnetic field produced by the fMRI. Some of this problems have been solved with modern recording techniques allowing the simultaneously recording of fMRI and EEG and by reducing the scanner noise (c.f. Wehrle et al., 2007, Czisch et al, 2002). For positron emission tomography (PET) the problems are less problematic and therefore more sleep studies have been done by PET scans than by fMRI. In general, the fMRI and PET studies demonstrated that brain activation patterns are different in different sleep stages and differ from brain activation in wakefulness (e.g. Braun et al., 1997; Dang-Vu, T. et al. 2006, Lövblad et al., 1999; Maquet et al., 1997).
Hong, Gillin, Dow, Wu, and Buchsbaum (1995) using positron emission tomography (PET) observed that eye movements during REM sleep involve the same cortical areas that control waking saccadic eye movement and attention. In a study by Maquet et al. (2000) participants were trained in visuomotor task at the evening. In the following sleep period REM sleep was recorded by PET scans. The PET scans showed a high activation of the motor areas in the central cortex. The results were interpreted by the reactivation of learning-related cerebral activity during post-training sleep, suggesting that sleep plays a role in the offline processing and consolidation of memory (Peigneux et al., 2003). Unfortunately dream content was not elicited in this or similar studies investigating sleep-dependent memory consolidation.
3. Autonomic Responses
3.1. Cardiovascular activity
There are a few studies from dream research that investigated the relationship between dream content and autonomic parameters, especially cardiorespiratory parameters. Hobson, Goldfrank, and Snyder (1965) found a positive correlation between global dream content ratings (including ratings of emotion, physical activity, and vividness) and respiration rate and respiration rate variability. Hauri and Van de Castle (1973) differentiated the dream content ratings in emotionality, physical activity and involvement, and found that physical activity is correlated to heart rate variability. However, a large number of correlation coefficients were computed and 17 of 100 coefficients reached significance (p < .05) and a correction for multiple comparisons was not attempted. Baust and Engel (1971) found that large respiration amplitude correlated to intensive active participation in the dream. On the other hand, high variability of the respiration frequency was associated – in contrast to the findings from Hobson et al. – with little active participation in the dream. No correlation was found for heart rate to dream content in this study. Overall, the findings in this area are inconsistent and conclusions about the relation between dream emotions or other dream characteristics and physiological parameters cannot yet be made.
In a pilot study, LaBerge, Greenleaf, and Kedzierski (1983) showed a correspondence between subjectively experienced sexual activity during REM lucid dreaming and several autonomic parameters such as, respiration rate, skin conduction, vaginal EMG and vaginal pulse amplitude. The parameters increased significantly during experienced lucid dream orgasm, but contrary to expectations, heart rate increased only slightly; the increase was not significant. In another study by LaBerge and Dement (1982) it was shown that lucid dreamers are able to voluntary control their respiration during lucid REM dreaming. In a study by Erlacher and Schredl (in Press) proficient lucid dreamers were instructed to carry out specific tasks (counting vs. performing squats) while lucid dreaming. During the night heart rate and respiration rate were measured continuously. The results showed an increase of heart rate while performing squats in the lucid dream. The results for respiration rate were less clear but showed the expected changes with higher respiration rates while squatting in the dream. In Figure 2, a recording of successfully carried out squats is depicted.
4. Time aspects
4.1. Dream time and REM sleep duration
The relationship between subjectively estimated time in dreams and real time has a long history in dream research. The following dream example reported by the French sleep and dream researcher Alfred Maury (1861) raised the question whether the recalled dream is really a recollection of experiences made during sleep or is possibly generated like a flash during the process of awakening. Maury reported a long and intense dream about the French revolution which ended with the dreamer in the guillotine and the sleeper waking up with a piece of his wooden bed top having fallen on his neck. Because of the logical line of dream action, Maury (1861) hypothesized that the dream was generated backwards by the arousing stimulus.
Nowadays, the hypothesis is widely accepted that the subjectively experienced time in dreams corresponds with the actual time (overview: Schredl, 2000). This relationship was first experimentally demonstrated by Dement and Kleitman (1957). In this study, the participants were awakened in a random order either after 5 or 15 minutes of REM sleep. After awakening, participants were asked to estimate whether the elapsed sleep interval was 5 or 15 minutes. From 111 awakenings, 83 % judgments were correct. Furthermore, the elapsed time of the REM period correlated with the length of the dream report (from r=.40 to r=.71). The latter findings were replicated by Glaubman and Lewin (1977), as well as by Hobson and Stickgold (1995). Rosenlicht, Maloney, and Freiberg (1994) found only small differences between time of REM sleep and the reported length of dreams.
In a pilot study, LaBerge (1985) showed that time intervals for counting from one to ten in lucid dreams (by counting from onethousand-and-one to one-thousand-and-ten) are close to the time intervals for counting during wakefulness. In a study by Erlacher and Schredl (2004) the time intervals for two different activities (performing squats and counting) were compared between lucid dreams and wakefulness. The results for counting replicates the finding of LaBerge’s (1985), that time intervals for counting were quite similar in lucid dreams and in wakefulness but performing squats required 44.5 % more time in lucid dreams than in the waking state. The authors speculated that this disproportional effect was caused by the modality of the activity (cognitive vs. motor activity).
5. Lucid dreaming and motor learning
Further evidence for the hypothesis that dreamed actions are equivalent to actual actions are provided by recent findings from sport science. There is growing evidence that lucid dreams can be used for practicing motor skills and that this practice has positive effects on subsequent performance in wakefulness (cf. Erlacher, 2005). Practice in lucid dreams is comparable to mental practice which is well known in sport theory and sport practice (e.g. Smith & Lee, 2005). This practice in lucid dreams is a novel type of mental rehearsal whereas a person is using the dream state to consciously practice specific tasks without waking up. For both types of menta rehearsal, movements are simulated with an imagined body on a cognitive level whereas the physical body remains still. In contrast to mental practice which is performed during wakefulness, practice in lucid dreams is applied while sleeping (c.f. Erlacher, 2005). For mental practice several meta-analysis (c.f. Driskell, Copper & Moran, 1994) demonstrated that in general mental practice enhances performance. It seems plausible that practice in lucid dreams shows also beneficial effects. But in contrast to vast research on mental practice, for practice in lucid dreams the empirical basis is rather small which can be explained by the fact that lucid dreaming is a rather unknown phenomena in the scientific sports community.
In several anecdotal reports (Erlacher, 2005; LaBerge & Rheingold, 1990; Tholey, 1990) amateur and professional athletes claimed that they use lucid dreams to improve their performance in the waking state. LaBerge and Rheingold (1990) reported of several amateur athletes who improved their skills during lucid dreams, e.g. a long distance runner who practiced his running technique, a tennis novice who learned his tennis serve and a woman who enhanced her skating skills. Tholey (1990) who was a German sport psychologist and a pioneer in lucid dream research provided further examples of professional athletes (Alpine skiing, equestrian and martial arts) who used lucid dream practice on a frequent base not only on accidental occasions.
In addition to his research interests in lucid dreaming, Tholey was himself a highly skilled lucid dreamer. In an interview he reported his own experiences in lucid dream practice: “Not every single movement I do practice in my lucid dreams. In lucid dreams I rather work on my body feeling and the orientation in the three dimensional space. For example I do somersaults and feel every single muscle twitch in my dreamed body.” (Mechsner, 1994). With his lucid dream practice he improved several sport techniques in wakefulness (Erlacher, 2005). Further anecdotal reports from professional athletes were collected by Erlacher (2005), for example:
Spring board diving (female, 33): „I practice complex twists and somersaults in my lucid dreams. When I do my lucid dream practice the movements feel real but if I want to I can slow down the whole sequence to focus on important details of the dive.”
Sprint training (male, 38): „Then I start to push my legs to the back and to concentrate on my feet and not only to lift my knees. Immediately I feel a force in my hips which brings me forward. The hips are now much better involved in my sprint than before.“
Winter sport (male, 26): „I dreamed lucidly about snowboarding, I was in fun park and I practice several tricks on my board. I couldn‘t do those tricks in waking life but the practice in my dreams helped to get better.“
Up to now there are very few studies which investigated the possible effects of practicing in lucid dreams in a systematic way. In a qualitative study by Tholey (1981, 1984), lucid dreamers were instructed to perform different complex sport skills, like skiing or gymnastics which the participants already know from waking life, in their lucid dreams. The participants reported that they had no difficulties to perform those complex sport skills in their lucid dreams. This ability is a prerequisite for lucid dream practice. Furthermore, the participants reported that the movements were accompanied by a pleasant feeling in the dream and that the movements improved due to the practice for both the dream state and the waking state. But in this study, performance after dreaming was not measured.
In a quasi-experimental pre-post design study conducted by Erlacher (2005) participants were asked to practice an aiming task in their lucid dreams. For the aiming task the participants had to toss a 10-cent Euro coin into a cup. The pre test in the evening and post test in the morning consisted of 20 tosses each. The results showed a significant increase of hit rate from pre test to post test for the group which practiced the coin tossing task in their lucid dreams but no increase was found for the other group. Even though the experimental design is not able to exclude confounding variables such as motivation, the results of this study indicate that practice in lucid dreams enhances performance in wakefulness.
To summarize, first, the anecdotal reports presented in this section demonstrated that lucid dreams can be used for practice of even complex sport skills (e.g. somersaults) within the dream. Second, it was shown that practice in lucid dreams have positive effects on actual performance in wakefulness. Even though those results have to be considered as preliminary and need to be replicated in additional samples, they fit well into theory of neural simulation of action. For motor imagery Jeannerod (1994) concluded that the positive effects of imagining movements on motor learning (e.g. Munzert & Hackfort, 1994) suggest a close functional equivalence between motor imagery and motor preparation since the cognitive processes must interfere at some level. The same holds true for practice in lucid dreams: the enhancement of the performance rate can be explained by the improvement of the neural networks which is the same for wakefulness and for dreams (c.f. Erlacher, 2005).
6. Discussion
The studies reviewed in the present article demonstrate that REM (lucid) dreamed actions are comparable like imagination during mental practice to actual actions and that it is plausible that the dreamed action share to some extend the same neural substrate on a cognitive level as if the action is carried out in the waking state. Those findings were presented for central nervous activity, autonomic responses and time aspects – as suggested by Decety (1996) and fit well into the theory of neural simulation of action (Jeannerods, 2001). Therefore in this paper evidence was gathered to show that dreamed actions indeed are S-state as proposed by Jeannerod (2001).
The strongest support was derived from the research on the central nervous activity on REM dreams (e.g. eye movements, EMG activity). In contrast, the studies for the autonomic responses and the time aspects provided inconsistent results. For example, the findings for estimating time intervals by counting support the hypothesis of a correspondence between time duration in lucid dreams and in the waking state. but the findings regarding motor activities, like performing squats, which required more time in lucid dreams than in wakefulness, did not.
The mixed results in dream research might be explained by methodological limitations like the large variability of the dream content, i.e., a large number of participants have to be studied in order to extract substantial but presumably small correlations. Overall, the number of studies in this field is small and in lucid dream research the sample often consists of only a few participants because it is difficult to recruit many proficient lucid dreamers.
Another point is that research focused on REM dreams but not on NREM dreams. As there is no specific muscle atonia present during NREM sleep, it seems very promising to link dreamed actions from NREM dreams to physiological parameters recorded prior to the awakening. Also for lucid dreaming a study by Dane (1986) showed that lucid dream experience also can occur during NREM sleep. To integrate those findings will be a challenging task for future research.
The findings from lucid dreaming and motor learning have beside their theoretical interest also practical implications for sports. Practice in lucid dreams might be a novel type for athletes of covert rehearsal, comparable to mental practice which is well known in sport theory and sport practice (e.g. Smith & Lee, 2005). For practice in lucid dreams and mental rehearsal, movements are simulated with an imagined body on a cognitive level whereas the physical body remains still. Lucid dream practice would provide professional athletes the additional chance to use the night time to work out specific skills or to rehears a competition. Further applications would be for athletes who suffer an injury and are unable to physically practice. And finally, lucid dream practice would give athletes the opportunity to practice skills which are dangerous (cliff diving) in wakefulness or are strongly dependent on environmental settings (Alpine Skiing in summer). That this practice will interfere negative with natural processes in sleep seems unlikely because even good lucid dreamer will have lucid dreams on small percentage compared to total amount of nightly REM sleep (Schredl, 2007).
7. Future directions
Despite the clear evidence of a relationship between physiological processes and dream content, a lot of questions remained unresolved and should be addressed in future research.
First, further research is needed to clarify the inconsistent results especially for the time aspects during dreams. Erlacher and Schredl (2004) suggested that one possible factor causing the different time intervals might be the different modalities of the tasks (e.g., motor vs. cognitive activities) and another factor might be that the duration of the tasks were unequal. To clarify if the modality or the length of the task caused the disproportional effects, in further studies different tasks (cognitive vs. motor) and time intervals (e.g. 10, 20, 30 seconds) should be examined.
Second, further research using modern neuroimaging techniques will be very promising. Comparing dream reports after awakening with the brain activation patters in this period of sleep will shed more light on the question whether the brain regions that are necessary for carrying out actions or processing incoming information in the waking state are also active while dreaming these actions or processes. Even though modern neuroimaging techniques have their problems the latest result form demonstrate that those problems are solvable (cf. Dang-Vu et al., 2006). One of the most interesting tasks for future research will be to study lucid dreaming by modern neuroimaging techniques. Even though it will be a challenging task for a participant to have lucid dreams in a fMRI scanner, studies from motor imagery have already demonstrated that this approach bears fruitful insights into the neural structures on the cortex level (cf. Lacourse, Orr, Cramer, & Cohen, 2005).
Third, research on lucid REM dreams have been proven to be very effective for investigating specific questions because lucid dreamers can be instructed to perform different tasks in the dream state and mark those events by eye movements in the EOG recording . The studies presented demonstrated that also complex tasks can be accomplished by lucid dreamers during their lucid dreams. Beside studying lucid dreams with neuroimaging techniques another interesting perspective will be to study the interaction between the lucid dreamer and external stimuli. As shown by Strelen (2006) it is possible for participants to “hear” acoustic stimuli from the external “world” and to successfully discriminate two different tones. This is a kind of simple communication between the research in the sleeping lab and the lucid dreamer in his dream world. To further extend this communication process will be very promising research area.
Fourth, the findings should be extended to for NREM sleep. Although Antrobus (1983) has shown that these reports were more thoughtlike and contained less often images, the variability is considerable so that some NREM reports can not be distinguished from REM reports. The question arising is how is it possible that visual imagery is present and eye movements are absent. Unfortunately, research in the mind-body interaction during NREM sleep is scarce and should be pursued in the future.
Finally, for a sport perspective it would be interesting to collect data from professional athletes concerning the knowledge about lucid dreaming and the possibility of practice in lucid dreams. It might be possible that already a small but substantial part of athletes already use lucid dreaming to improve their performance. This might be true since in a representative survey in Austria (N = 1000), 26 % of the participants stated that they had experienced a lucid dream at least once (Stepansky et al., 1998). It would be interesting to see if their are sports which might be more promising for lucid dreamer than others, e.g. sports were technique is dominant in contrast to sports were conditional factors are important. Furthermore it would be interesting to provide professional athletes induction techniques to introduce them to lucid dream practice. But this will be a further challenging task for further research to find good induction techniques to reliable induce lucid dreams in a night; not only for sport but also for lucid dream research in general.
Literature
Antrobus, J. S. (1983). REM and NREM sleep reports: comparison of word frequencies by cognitive classes. Psychophysiology, 20, 562-568.
Baust, W., & Engel, R. R. (1971). The correlation of heart and respiratory fre-quency in natural sleep of man and their relation to dream content. Electroencephalography and Clinical Neurophysiology, 30, 262-263.
Birbaumer, N., & Schmidt, R. F. (1996). Biologische Psychologie ( 3. ed.). Berlin: Springer.Braun, A. R., Balkin, T. J., Wesensten, N. J., Carson, R. E., Varga, M., Baldwin, P., Selbie, S., Belenky, G., & Herscovitch, P. (1997). Regional cere-bral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain, 120, 1173-1197.
Czisch, M., Wetter, T. C., Kaufmann, C., Pollmächer, T., Holsboer, F., & Auer, D. P. (2002). Altered processing of acoustic stimuli during sleep: reduced auditory activation and visual deactivation detected by a combined fMRI/EEG study. Neuroimage, 16, 251-258.
Dane, J. (1986). Non-REM lucid dreaming. Lucidity Letter, 5, 133-145.
Dang-Vu, T.T., Desseilles, M., Albouy, G., Darsaud, A., Gais, S. ,Rauchs, S. ,Schabus, V., Sterpenich, V., Vandewalle, G., Schwartz, S., Maquet, P. (2006). Dreaming: a neuroimaging view. Schweizer Archivs für Neurologie und Psychiatrie, 156, 415-425.
Decety, J. (1996). Do imagined and executed actions share the same neural substrate? Cognitive Brain Research, 3, 87-93.
Decety, J., Jeannerod, M., Germain, M., & Pastene, J. (1991). Vegetative re-sponse during imagined movement is proportional to mental effort. Behavioural Brain Research, 42, 1-5.
Dement, W. C. (1974). Some must watch while some must sleep. San Fran-cisco: W. H. Freeman.
Dement, W., & Kleitman, N. (1957) The relation of eye movements during sleep to dream activity: an objective method for the study of drea-ming. Journal of Experimental Psychology, 53, 339-346.
Dement, W., & Wolpert, E. A. (1958). The relation of eye movements, body motility, and external stimuli to dream content. Journal of Experi-mental Psychology, 55, 543-553.
Driskell, J. E., Copper, C., & Moran, A. (1994). Does mental practice enhance performance? Journal of Applied Psychologie, 79, 481-492.
Eisensehr, I., Linke, R., Noachtar, S., Schwarz, J., Gildehaus, F. J., & Tatsch, K. (2000). Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder Comparison with Parkinson‘s disease and controls. Brain, 123, 1155-1160.
Erlacher & Schredl (in press). Cardiovascular responses to dreamed ”physical exercise” during REM lucid dreaming. Dreaming.Erlacher, D. & Schredl, M. (2004). Required time for motor activities in lucid dreams. Perceptual and Motor Skills, 99, 1239-1242.
Erlacher, D. (2005). Motorisches Lernen im luziden Traum: Phä-nomenologische und experimentelle Betrachtungen.
Erlacher, D., Schredl, M. & LaBerge, S. (2003). Motor area activation during dreamed hand clenching: A pilot study on EEG alpha band. Sleep and Hypnosis, 5, 182-187.
Etevenon, P., & Guillou, S. (1986). EEG cartography of a night of sleep and dreams. A longitudinal study with provoked awakenings. Neuropsy-chobiology, 16, 146-151.
Fenwick, P., Schatzmann, M., Worsley, A., Adams, J., Stone, S., & Backer, A. (1984). Lucid dreaming: correspondence between dreamed and actual events in one subject during REM sleep. Biological Psycho-logy, 18, 243-252.
Firth, H., & Oswald, I. (1975). Eye movements and visually active dreams. Psychophysiology, 12, 602-606.Foulkes, D. (1962). Dream reports from different stages of sleep. Journal of Abnormal and Social Psychology, 65, 14-25.
Gardner, R., Grossman, W. I., Roffwarg, H. P., & Weiner, H. (1975). The relati-onship of small limb movements during REM sleep to dreamed limb action. Psychosomatic Medicine, 37, 147-159.
Gerne, M., & Strauch, I. (1985). Psychophysiological indicators of affect pattern and conversational signals during sleep. In W. P. Koella, E. Rüther, & H. Schulz (Eds.), Sleep 1984 (pp. 367-369). Stuttgart: Gus-tav Fischer Verlag.
Grossman, W. I., Gardner, R., Roffwarg, H. P., Fekek, A. F., Beers, L., & Weiner, H. (1972). Relation of dreamed to actual movement. Psychophysio-logy, 9, 118-119.
Glaubman, H., & Lewin, I. (1977). REM and dreaming. Perceptual and Motor Skills, 44, 929-930.
Hauri, P., & Van de Castle, R. L. (1973). Psychophysiological parallels in dreams. Psychosomatic Medicine, 35, 297-308.
Hearne, K. (1983). Lucid dream induction. Journal of Mental Imagery, 7, 19-24.
Hearne, K. M. (1978). Lucid dreams: an electrophysiological and psychologi-cal study. University of Liverpool, Liverpool.
Heuer, H. (1985). Wie wirkt mentale Übung? Psychologische Rundschau, 36, 191-200.
Hobson, J. A., & Stickgold, R. (1995) The conscious state paradigm: a neu-rocognitive approach to waking, sleeping, and dreaming. In M. S. Gazzaniga (Ed.), The cognitive neurosciences (pp 1373-1389).
Hobson, J. A., Goldfrank, F., & Snyder, F. (1965). Respiration and mental acti-vity in sleep. Journal of psychiatric research, 3, 79-90.
Hobson, J. A., Pace-Schott, E. F., & Stickgold, R. (2000). Dreaming and the brain: Toward a cognitive neuroscience of conscious states. Beha-vioral and Brain Sciences, 23, 793-842.
Hofer, S. (1987). Emotionalität im Traum und EMG der Gesichtsmuskeln. Uni-versität Zürich: Unpublished Lizentiatsarbeit.
Holzinger, B., LaBerge, S., & Levitan, L. (2006). Psychophysiological correla-tes of lucid dreaming. Dreaming, 16, 88-95.
Hong, C. C.-H., Jin, Y., Potkin, S. G., Buchsbaum, M. S., Wu, J., Callaghan, G. M., Nudleman, K. L., & Gillin, J. C. (1996). Language in dreaming and regional EEG alpha power. Sleep: Journal of Sleep Research & Sleep Medicine, 19, 232-235.
Jeannerod, M. (1994). The representing brain: Neural correlates of motor in-tention and imagery. Behavioral and Brain Science, 17, 187-245.
Jeannerod, M. (2001). Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage, 14, 103-109.
Jouvet, M. (1979). What does a cat dream about? Trends in Neurosciences, 2, 280-282.
Jouvet, M. (1994). Die Nachtseite des Bewußtseins. Warum wir träumen. Hamburg: Reinbek.
Jouvet, M., & Delorme, F. (1965). Locus coeruleus et sommeil paradoxal. Comptes Rendus des Seances et Memoires de la Societe de Bio-logie, 159, 895-899.
Keefauver, P. S., & Guilleminault, C. (2000). Sleep terrors and sleepwalking. In M. H. Kryger & T. Roth & W. C. Dement (Eds.), Principles and Practi-ce of Sleep Medicine (4. ed., pp. 567-573). Philadelphia: Saunders.
LaBerge, S. (1980). Lucid dreaming: An exploratory study of consciousness during sleep. Stanford University, Palo Alto, CA.
LaBerge, S. (1985). Lucid dreaming. Los Angeles: Tarcher.
LaBerge, S., & Dement, W. C. (1982a). Lateralization of alpha activity for dreamed singing and counting during REM sleep. Psychophysio-logy, 19, 331-332.
LaBerge, S., & Dement, W. C. (1982b). Voluntary control of respiration during lucid REM dreaming. Sleep research, 11, 107.
LaBerge, S., & Rheingold, H. (1990). Exploring the world of lucid dreams. New York: Ballantine.
LaBerge, S. & Zimbardo, P.G. (2000). Smooth tracking eye-movements discriminate both dreaming and perception from imagination. Paper presented at the Toward a Science of Consciousness Conference, Tucson, April 10-15.
LaBerge, S., Greenleaf, W., & Kedzierski, B. (1983). Physiological responses to dreamed sexual activity during REM sleep. Psycholphysiology, 19, 454-455.
LaBerge, S., Nagel, L. E., Dement, W. C., & Zarcone, V. P. (1981). Lucid dre-aming verified by volitional communication during REM sleep. Per-ceptual and Motor Skills, 52, 727-732.
Lacourse, M. G., Orr, E. L. R., Cramer, S. C., & Cohen, M. J. (2005). Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage, 27, 505-519.
Ladd, G. T. (1892). Contribution to the psychology of visual dreams. Mind, 1, 299-304.
Maquet, P., Degueldre, C., Delfiore, G., Aerts, J., Peters, J.-M., Luxen, A., & Franck, G. (1997). Functional neuroanatomy of human slow wave sleep. Journal of Neuroscience, 17, 2807-2812.
Maury, A. (1861) Le sommeil et les reves. Paris: Didier.
McGuigan, F. J., & Tanner, R. G. (1971). Covert oral behavior during conversa-tional and visual dreams. Psychonomic Sciences, 23, 263-264.
Mechsner, F. (1994). Geschichten aus der Nacht. Geo, 2, 12-30.
Munzert, J. (2002). Temporal accuracy of mentally simulated transport move-ments. Perceptual and Motor Skills, 94, 307-318.
Munzert, J., & Hackfort, D. (1999). Individual preconditions for mental trai-ning. International Journal of Sport Psychology, 30, 41-62.
Niedermeyer, E. (1987). Sleep and EEG. In E. Niedermeyer (Ed.), Electroence-phalography: Basic principles, clinical applications, and related fields (2nd ed., pp. 153-166). Baltimore: Urban & Schwarzenberg.
Peigneux, P., Laureys, S., Fuchs, S., Destrebecqz, A., Collette, F., Delbeuck, X., Phillips, C., Aerts, J., Del Fiore, G., Degueldre, C., Luxen, A., Cleeremans, A., & Maquet, P. (2003). Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage, 20, 125-134.
Pfurtscheller, G. & Neuper, Ch. (1997). Motor imagery activates primary sen-sorimotor area in humans. Neuroscience Letters, 239, 65-68.
Pfurtscheller, G. (1992). Event-related synchronization (ERS): an electrophy-siological correlate of cortical areas at rest. Electroencephalography and Clinical Neurophysiology, 83, 62-69.
Roffwarg, H. P., Dement, W. C., Muzio, J. N., & Fischer, C. (1962). Dream imagery: relationship to rapid eye movements of sleep. Archives of general psychiatry, 7, 235-258.
Roland, P. E., Larsen, B., Lassen, N. A., & Skinhoj, E. (1980). Supplementary motor area and other cortical areas in organization of voluntary mo-vements in man. Journal of Neurophysiology, 43, 118-136.
Rosenlicht, N., Maloney, T., & Freiberg, I. (1994) Dream report length is more dependent on arousal level than prior REM duration. Brain Research Bulletin, 34, 99-101.
Schenck, C. H., & Mahowald, M. W. (1996). REM sleep parasomnias. Neurologic Clinics, 14, 697-720.
Schenck, C. H., & Mahowald, M. W. (2002). REM sleep behavior disorder: Cli-nical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep, 25, 120-138.
Schenck, C. H., Bundlie, S. R., Ettinger, M. G., & Mahowald, M. W. (1986). Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep, 9, 293-308.
Schredl, M. (2000) Body-mind interaction: dream content and REM sleep physiology. North American Journal of Psychology, 2, 59-70.
Schredl, M. (2007). Träume. Berlin: Ullstein. Schredl, M., & Erlacher, D. (2004). Lucid dreaming frequency and personality. Personality and Individual Differences, 37, 1463-1473.
Shimizu, A., & Inoue, T. (1986). Dreamed speech and speech muscle activity. Psychophysiology, 23, 210-214.
Smith & Lee (2005). Motor Control and Learning. A Behavioral Emphasis. Campaign: Human Kinetic.
Stepansky, R., Holzinger, B., Schmeiser-Rieder, A., Saletu, B., Kunze, M., & Zeitlhofer, J. (1998). Austrian dream behavior: results of a repre-sentative population survey. Dreaming, 8, 23-30.Stepansky et al., 1998;
Strelen, J. (2006). Akustisch evozierte Potentiale bei luziden Träumen. Unpu-blished doctoral thesis: University of Mainz.
Tholey, P. (1981). Empirische Untersuchungen über Klarträume. Gestalt The-ory, 3, 21–62.
Tholey, P. (1984). Sensumotorisches Lernen als Organisation des psychi-schen Gesamtfelds. In E. Hahn & H. Rieder (Eds.), Sensumotori-sches Lernen und Sportspielforschung (pp. 11-26). Köln: bsp.
Tholey, P. (1990). Applications of lucid dreaming in sports. Lucidity Letter, 9, 6-17.
Wehrle, R., Kaufmann, C., Wetter, T. C., Holsboer, F., Auer, D. P., Pollmächer, T., & Czisch, M. (2007). Functional microstates within human REM sleep: first evidence from fMRI of a thalamocortical network spe-cific for phasic REM periods. European Journal of Neuroscience, 25, 863-871.
Wolpert, E. A. (1960). Studies in psychophysiology of dreams: II. an electro-myographic study of dreaming. Archives of General Psychiatry, 2, 231-241.
Zadra, A. L., & Nielsen, T. A. (1996). EEG spectral analyses of REM nightmares and anxiety dreams. Paper presented at the Thirteenth International Conference of the Association for the Study of Dreams, Berkeley